Page 255 - Mirjam-Theelen-Degradation-of-CIGS-solar-cells
P. 255
The impact of atmospheric species
impact of water purged with N and O on ZnO:Al degradation was very small.
2
2
The degradation on complete CIGS cells showed similar effects: the samples exposed
to water purged with N and O were more or less stable as a function of time, while
2
2
the samples exposed to water purged with large quantities of CO degraded quickly.
2
However, unlike the ZnO:Al experiment, the unpurged water sample was also stable
in this case. The CO content might have been too low to have a large impact in this
2
case. For the experiments on complete solar cells, it is proposed that the dissolution of
part of the ZnO:Al is the driving force behind the loss of efficiency, while the formation
of species like Zn(OH) (CO ) (hydrozincite) will only have played a marginal role.
5
3 2
6
However, hydrozincite formation could still have occurred in case the dissolution was
avoided for a longer time, for example for the unpurged water samples. This would
have resulted in ZnO:Al layers that still have a closed structure, but have increased
potential barriers at the hydrozincite positions, for example at the grain boundaries,
leading to increased sheet resistance of the ZnO:Al (see chapter 6 and reference [1]).
Naturally, this would have led to an increased series resistance of the complete CIGS
solar cell as well.
Since the samples with gaps in the ZnO:Al and the highest hydroxide/water content
show the lowest conversion efficiency, it is proposed that these effects are detrimental
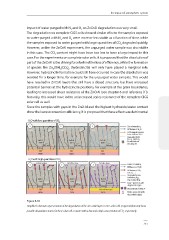
Figure 8.10:
Simplified schematic representation of the degradation of the zinc oxide layers in CIGS solar cells. (Top) and (bottom) show
possible degradation routes for these solar cells in water with a low and a high concentration of CO 2 respectively.
253
impact of water purged with N and O on ZnO:Al degradation was very small.
2
2
The degradation on complete CIGS cells showed similar effects: the samples exposed
to water purged with N and O were more or less stable as a function of time, while
2
2
the samples exposed to water purged with large quantities of CO degraded quickly.
2
However, unlike the ZnO:Al experiment, the unpurged water sample was also stable
in this case. The CO content might have been too low to have a large impact in this
2
case. For the experiments on complete solar cells, it is proposed that the dissolution of
part of the ZnO:Al is the driving force behind the loss of efficiency, while the formation
of species like Zn(OH) (CO ) (hydrozincite) will only have played a marginal role.
5
3 2
6
However, hydrozincite formation could still have occurred in case the dissolution was
avoided for a longer time, for example for the unpurged water samples. This would
have resulted in ZnO:Al layers that still have a closed structure, but have increased
potential barriers at the hydrozincite positions, for example at the grain boundaries,
leading to increased sheet resistance of the ZnO:Al (see chapter 6 and reference [1]).
Naturally, this would have led to an increased series resistance of the complete CIGS
solar cell as well.
Since the samples with gaps in the ZnO:Al and the highest hydroxide/water content
show the lowest conversion efficiency, it is proposed that these effects are detrimental
Figure 8.10:
Simplified schematic representation of the degradation of the zinc oxide layers in CIGS solar cells. (Top) and (bottom) show
possible degradation routes for these solar cells in water with a low and a high concentration of CO 2 respectively.
253