Page 253 - Mirjam-Theelen-Degradation-of-CIGS-solar-cells
P. 253
The impact of atmospheric species
ZnO:Al
10 4
H O/air 2 Cu(In,Ga)Se 2
2
H O
Intensity (counts) 10 2 Air Ref H O/O 2
3
10
Mo
2
2
2
10 1 H O/CO /N 2
10 0
0 1000 2000 3000
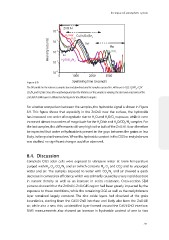
Figure 8.9: Sputtering time (seconds)
The OH profile for the reference samples (air and glovebox) and for samples exposed for 340 hours to H 2 O, H 2 O/O 2 , H 2 O/
CO 2 /N 2 and H 2 O/air. Since the morphology and also the thickness of the samples is varying, the start and end points of the
ZnO:Al/CIGS/Mo layers is different in this figure for the different samples.
For a better comparison between the samples, the hydroxide signal is shown in Figure
8.9. This figure shows that especially in the ZnO:Al near the surface, the hydroxide
has increased one order of magnitude due to H O and H O/O exposure, while it even
2
2
2
increased almost two orders of magnitude for the H O/air and H O/CO /N samples. For
2
2
2
2
the last samples, this difference is still very high in the bulk of the ZnO:Al. It can therefore
be expected that water or hydroxide is present in the gaps between the grains or less
likely, in the grains themselves. When the hydroxide content in the CIGS or molybdenum
was studied, no significant changes could be observed.
8.4. Discussion
Complete CIGS solar cells were exposed to ultrapure water at room temperature
purged with N , O , CO /N and air (which contains N, O and CO ) and to unpurged
2
2
2
2
2
2
2
water and air. The samples exposed to water with CO/N and air showed a quick
2
2
decrease in conversion efficiency, which was primarily caused by a very rapid decrease
in current density as well as an increase in series resistance. Cross-section SEM
pictures showed that the ZnO:Al/i-ZnO/CdS region had been greatly impacted by the
exposure to these conditions, while the remaining CIGS as well as the molybdenum
layer remained largely constant. The zinc oxide layers had dissolved at the grain
boundaries, starting from the CdS/i-ZnO interface and likely also from the ZnO:Al/
air, while also a new thin, unidentified layer formed around the CdS/i-ZnO interface.
SIMS measurements also showed an increase in hydroxide content of one to two
251
ZnO:Al
10 4
H O/air 2 Cu(In,Ga)Se 2
2
H O
Intensity (counts) 10 2 Air Ref H O/O 2
3
10
Mo
2
2
2
10 1 H O/CO /N 2
10 0
0 1000 2000 3000
Figure 8.9: Sputtering time (seconds)
The OH profile for the reference samples (air and glovebox) and for samples exposed for 340 hours to H 2 O, H 2 O/O 2 , H 2 O/
CO 2 /N 2 and H 2 O/air. Since the morphology and also the thickness of the samples is varying, the start and end points of the
ZnO:Al/CIGS/Mo layers is different in this figure for the different samples.
For a better comparison between the samples, the hydroxide signal is shown in Figure
8.9. This figure shows that especially in the ZnO:Al near the surface, the hydroxide
has increased one order of magnitude due to H O and H O/O exposure, while it even
2
2
2
increased almost two orders of magnitude for the H O/air and H O/CO /N samples. For
2
2
2
2
the last samples, this difference is still very high in the bulk of the ZnO:Al. It can therefore
be expected that water or hydroxide is present in the gaps between the grains or less
likely, in the grains themselves. When the hydroxide content in the CIGS or molybdenum
was studied, no significant changes could be observed.
8.4. Discussion
Complete CIGS solar cells were exposed to ultrapure water at room temperature
purged with N , O , CO /N and air (which contains N, O and CO ) and to unpurged
2
2
2
2
2
2
2
water and air. The samples exposed to water with CO/N and air showed a quick
2
2
decrease in conversion efficiency, which was primarily caused by a very rapid decrease
in current density as well as an increase in series resistance. Cross-section SEM
pictures showed that the ZnO:Al/i-ZnO/CdS region had been greatly impacted by the
exposure to these conditions, while the remaining CIGS as well as the molybdenum
layer remained largely constant. The zinc oxide layers had dissolved at the grain
boundaries, starting from the CdS/i-ZnO interface and likely also from the ZnO:Al/
air, while also a new thin, unidentified layer formed around the CdS/i-ZnO interface.
SIMS measurements also showed an increase in hydroxide content of one to two
251