Page 228 - Mirjam-Theelen-Degradation-of-CIGS-solar-cells
P. 228
Chapter 7
alkali-poor
alkali-rich
μm
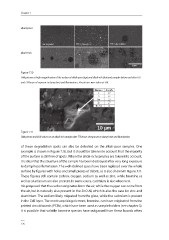
Figure 7.10
SEM pictures at high magnification of the surface of alkali-poor (top) and alkali-rich (bottom) samples before and after 165
and 778 hours of exposure to damp heat and illumination. The pictures were taken at 5kV.
10 µm
Figure 7.11
SEM picture and EDX values on an alkali-rich sample after 778 hours of exposure to damp heat and illumination
of these degradation spots can also be detected on the alkali-poor samples. One
example is shown in Figure 7.10, but it should be taken into account that the majority
of the surface is still free of spots. When the alkali-rich samples are taken into account,
it is clear that the structure of the sample has been destroyed after very long exposure
to damp heat illumination. The well-defined spots have been replaced over the whole
surface by figures with holes and small pieces of debris, as is also shown in Figure 7.11 .
These figures still contain carbon, oxygen, sodium as well as zinc, while bromine as
well as aluminium are also present. In some cases, cadmium is also observed.
It is proposed that the carbon originates from the air, while the oxygen can come from
the air, but is naturally also present in the ZnO:Al, which is also the case for zinc and
aluminium. The sodium likely migrated from the glass, while the cadmium is present
in the CdS layer. The most surprising element, bromine, can have originated from the
printed circuit boards (PCBs), which have been used as sample holders (see chapter 3).
It is possible that volatile bromine species have outgassed from these boards when
226
alkali-poor
alkali-rich
μm
Figure 7.10
SEM pictures at high magnification of the surface of alkali-poor (top) and alkali-rich (bottom) samples before and after 165
and 778 hours of exposure to damp heat and illumination. The pictures were taken at 5kV.
10 µm
Figure 7.11
SEM picture and EDX values on an alkali-rich sample after 778 hours of exposure to damp heat and illumination
of these degradation spots can also be detected on the alkali-poor samples. One
example is shown in Figure 7.10, but it should be taken into account that the majority
of the surface is still free of spots. When the alkali-rich samples are taken into account,
it is clear that the structure of the sample has been destroyed after very long exposure
to damp heat illumination. The well-defined spots have been replaced over the whole
surface by figures with holes and small pieces of debris, as is also shown in Figure 7.11 .
These figures still contain carbon, oxygen, sodium as well as zinc, while bromine as
well as aluminium are also present. In some cases, cadmium is also observed.
It is proposed that the carbon originates from the air, while the oxygen can come from
the air, but is naturally also present in the ZnO:Al, which is also the case for zinc and
aluminium. The sodium likely migrated from the glass, while the cadmium is present
in the CdS layer. The most surprising element, bromine, can have originated from the
printed circuit boards (PCBs), which have been used as sample holders (see chapter 3).
It is possible that volatile bromine species have outgassed from these boards when
226